Class 9 Atoms and Molecules Notes
Notes Important Questions NCERT Solutions MCQ Quiz – 1 MCQ Quiz – 2Atoms and Molecules Notes
Laws of chemical combination:-
- Law of conservation of mass:- It states that mass can neither be created nor be destroyed in a chemical reaction.
Or,
It states that the total mass of reactants is equal to total mass of products in a chemical reaction. - Law of constant proportion:- It states that in a chemical substance the elements are always present in constant proportion by mass.
Dalton’s atomic theory:-
According to Dalton’s atomic theory, all matter, whether an element, a compound or a mixture is composed of small particles called atoms.
Postulates of Dalton’s atomic theory:-
- All matter is made of very tiny particles called atoms.
- Atoms are invisible particles, which cannot be created or destroyed in a chemical reaction.
- Atoms of a given element are identical in mass and chemical properties.
- Atoms of different elements have different masses and chemical properties.
- Atoms combine in the ratio of small whole numbers to form compounds .
- The relative number and kinds of atoms are constant in a given compound.
One atomic mass unit:- One atomic mass unit is the mass unit equal to exactly one twelfth (1/12th) the mass of one atom of carbon-12.
Relative atomic mass:- The relative atomic mass of the atom of an element is defined as the average mass of the atom, as compared to 1/12th the mass of one carbon-12 atom.
Molecule:-
A molecule can be defined as the smallest particle of an element or compound that is capable of independent existence and shows all the properties of the substance.
Molecules of element:- Molecules of an element are constituted by the same type of atoms. Ex:- He, H2, Cl2, O2, P4 etc.
Molecules of compound:- The molecules of compound are constituted by atoms of different elements which are joined together in definite proportion. Ex:- H2O, CO2 etc.
Atomicity:-
The number of atoms present in a molecule is known as atomicity.
| Element | Atomicity |
| Argon | Monoatomic |
| Helium | Monoatomic |
| Sodium | Monoatomic |
| Oxygen | Diatomic |
| Hydrogen | Diatomic |
| Phosphorus | Tetra-atomic |
| Sulphur | Poly-atomic |
Ion:-
An atom or a group of atoms having some charge is called an ion.
Ex:- Na+, OH–, Mg2+ etc.
Polyatomic ion:- A group of atoms having some charge is called polyatomic ion. Ex:- SO42-, NH4+ etc.
Cation:- A positively charged ion is called cation. Ex:- H+, Mg2+, etc.
Anion:- A negatively charged ion is called anion. Ex:- Cl–, OH–, etc.
Valence electron:- The number of electrons present in the outermost shell of an element is known as its valence electron.
Valency:- The combining capacity of an element is known as its valency.
Binary compounds:- The simplest compounds which are made up of two different elements are called binary compounds. Ex:- H2O, CO2 etc.
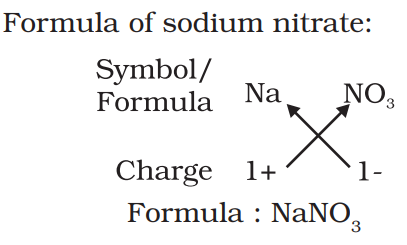
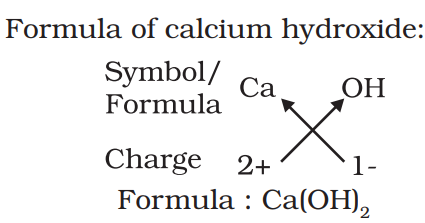
Chemical formula:-
The symbolic representation of the composition of a compound is known as its chemical formula. Ex:- H2O, CO2 etc.
Rules to write chemical formula:-
- The valencies or charges on the ion must balance.
- When a compound consists of a metal and a non-metal, the name or symbol of the metal is written first. For example: calcium oxide (CaO).
- In compounds formed with polyatomic ions, the ion is enclosed in a bracket before writing the number to indicate the ratio.
Molecular mass:- The sum of the atomic masses of all the atoms in a molecule of the substance is known as its molecular mass.
Formula unit mass:- The sum of the atomic masses of all atoms in a formula unit of a compound is known as its formula unit mass.
- Formula unit mass is calculated in the same manner as we calculate the molecular mass.
- The only difference is that we use the word formula unit for those substances whose constituent particles are ions.
Mole concept:-
- One mole:- One mole of any species (particles) is that quantity in number having a mass equal to its atomic or, molecular mass in grams.
- 1 mole = 6.022 x 1023 number of particles = Relative atomic/molecular mass in grams
- Avogadro number (No) = 6.022 x 1023
- Number of moles = Given mass/Molar mass
n = m/M - Number of moles = Given number of particles/Avogadro number
n = N/No - m/M = N/No


Comments