Class 10 – Periodic Classification Of Elements – Previous Years Questions
Previous Years Questions Notes Important QuestionsPeriodic Classification Of Elements – Previous Years Questions
- Write the atomic numbers of two elements ‘X’ and ‘Y’ having electronic configurations 2, 8, 2 and 2, 8, 6 respectively. [CBSE 2014] [1 Marks]
- State the common characteristics of the following elements:
Boron, Silicon, Germanium and Arsenic [CBSE 2020] [1 Marks] - An element X with atomic number 12 forms a compound with element Y with atomic number 17. The formula of compound formed is
- XY
- XY2
- X2Y
- X2Y3 [CBSE 2020] [1 Mark]
- An element X is forming acidic oxide. Its most probable position in the modern periodic table is
- Group 1 and period 3
- Group 16 and Period 3
- Group 17 and Period 3
- Group 2 and Period 3 [CBSE 2020] [1 Mark]
- State the periodic law on which the Modern Periodic Table is based. [CBSE 2020] [1 Marks]
- Study the following table in which the positions of six elements A, B, C, D, E and F are shown as they are in the modern periodic table:
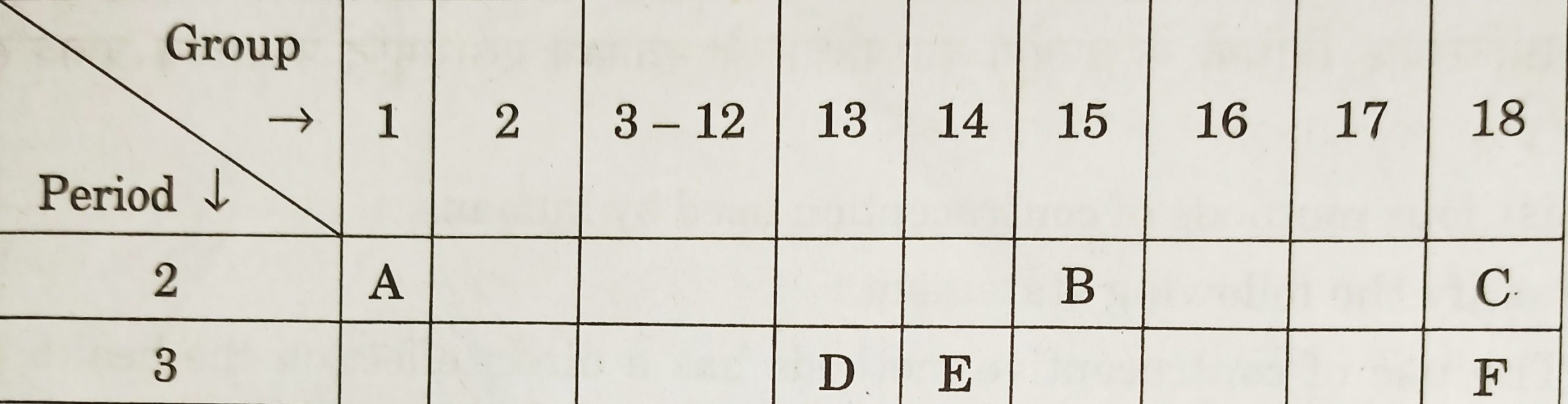
On the basis of the above table, answer the following questions :- Name the element which forms only covalent compounds.
- Name the element which is a metal with valency three.
- Name the element which is a non-metal with valency three.
- Out of D and E, Which is bigger in size and why ?
- Write the common name for the family to which the elements C and F belong. [CBSE 2014] [3 Marks]
- What is meant by ‘group’ in the modern periodic table? How do the following change on moving from top to bottom in a group?
- Number of valence electrons
- Number of occupied shells
- Size of atoms
- Metallic characters of elements
- Effective nuclear charge experienced by valence electrons [CBSE 2014] [3 Marks]
- An element ‘X’ belongs to third period and group 16 of the Modern Periodic Table.
- Determine the number of valence electrons and the valency of ‘X’.
- Molecular formula of the compound when ‘X’ reacts with hydrogen and write its electron dot structure.
- Name the element ‘X’ and state weather it is metallic or non-metallic. [CBSE 2016] [3 Marks]
- Three elements ‘X’ ‘Y’ and ‘Z’have atomic numbers 7, 8 and 9 respectively .
- State the positions (Group number and period number both) in the Modern Periodic Table.
- Arrange these elements in the decreasing order of their atomic radii.
- Write the formula of compound formed when ‘X’ combines with ‘Z’. [CBSE 2016] [3 Marks]
- .
- Name the element with atomic number 12.
- In which group it is placed?
- In which period it is positioned?
- Write down its electronic configuration. [CBSE 2017] [3 Marks]
- “Atomic number of an element is considered to be more appropriate parameter than its atomic mass for a chemist.” Take the example of the element X (atomic number 13) to justify this statement. [CBSE 2019] [3 Marks]
- .
- How is valency of an element determined from the electronic configuration of its atom?
- Determine the valency of an element X whose atomic number is 15. [Cbse 2019] 3 Marks]
- From the elements Li, K, Mg, C, Al, S identify the
- elements belonging to the same group.
- element which has the tendency to lose two electrons.
- element which prefers sharing of electrons to complete its octet.
- most metallic element.
- element that forms acidic oxide.
- element that belongs to group 13. [CBSE 2020] [3 Marks]
- .
- List any two distinguishing features between Mendeleev’s Periodic Table and the Modern Periodic Table.
- With the help of an example, explain Dobereiner’s Triads.
- State modern periodic law. [CBSE 2020] [3 Marks]
- The formulae of oxides of two elements X and Y are XO and Y2O3 respectively.
- Find the valencies of X and Y.
- Identify the groups in which they would be placed in the modern periodic table.
- Name one more element belonging to each of these groups. [CBSE 2017] [5 Marks]
- .
- The modern periodic table has been evolved through the early attempts of Dobereiner, Newland and Mendeleev. List one advantage and one limitation of all the three attempts.
- Name the scientist who first of all showed that atomic number of an element is a more fundamental property than its atomic mass.
- State modern periodic law. [CBSE 2018] [5 Marks]


Comments